The general expression for the change in. Total entropy at the end 214 2699 3538 J K-1.
How To Calculate Entropy Changes Liquids Solids And Phase Changes Youtube
The Clausius equation introduces the measurement of entropy change.
. You ended up with 1 mole of carbon dioxide and two moles of liquid water. In my 12th standard book the formula for entropy change is given as Δ S q reversible T. Under conditions of constant pressure and temperature the former is the energy released by the system into its environment traditionally represented by ΔH times 1 T and the latter is Δ S.
The Gibbs free energy change of the system the enthalpy change. H 2 g F 2 g 2HF g Answer. Δ S S B S A A B d q rev T A B d q irrev T.
Calculate the entropy change for the following reaction using the table of entropy values. The change in entropy is then the inverse of the temperature integrated over the change in heat transfer. This expression becomes via some steps the Gibbs free energy equation for reactants and products in the system.
For any such process. Delta S S f - S i. Total starting entropy 186 2205 596 J K-1 mol-1.
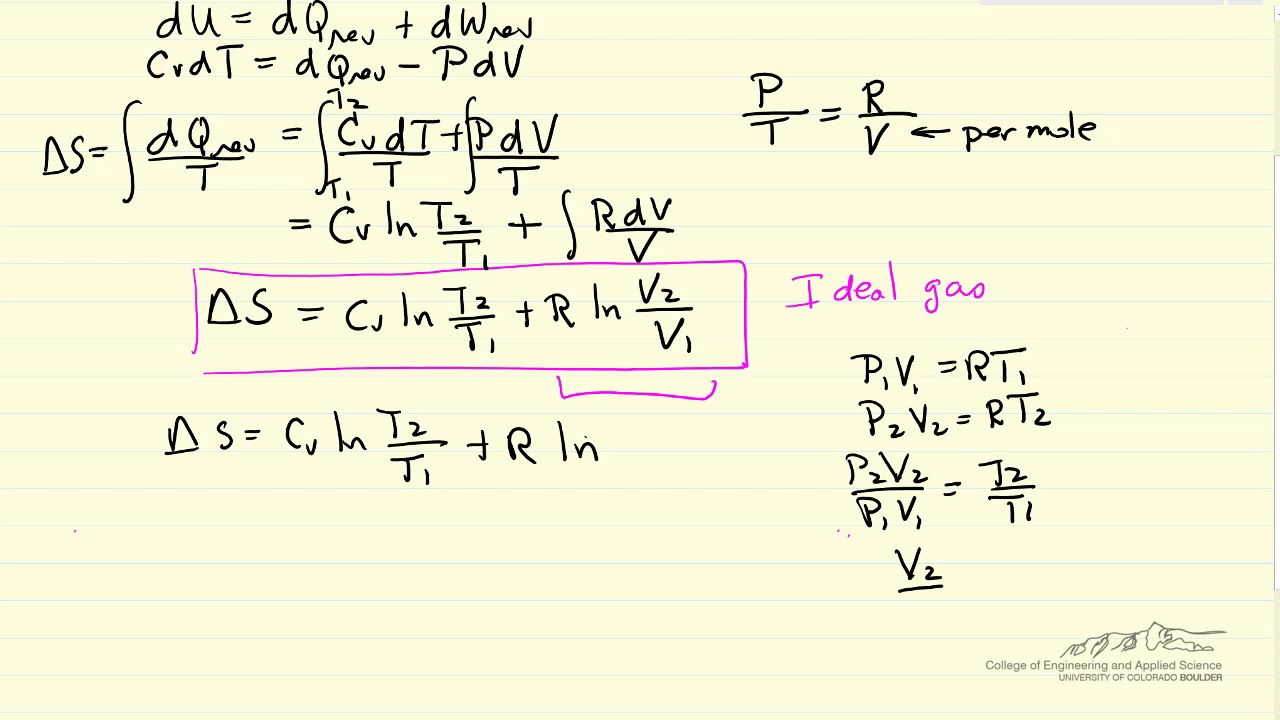
A system at higher temperatures will have greater randomness than a system at a lower. Entropy change states the direction and quantifies the magnitude of small changes ie. For gases there are two possible ways to evaluate the change in.
Entropy is typically considered a function of temperature and either volume or pressure. If we add the same quantity of heat at a higher temperature and lower temperature randomness will be maximum at a lower temperature. The change in entropy of the surroundings after a chemical reaction at constant pressure and temperature can be expressed by the formula ΔS surr -ΔHT where ΔS surr is.
There are also phase changes for these systems. S q reviso T. During chemical reactions if reactants break into more products then entropy also increases.
Formula of entropy change. The entropy formula is given as. Entropy is also a measure of the molecular disorder or randomness of a.
So we need to calculate the entropy change with a different approach than the solids liquids and ideal gases. Entropy is the measure of a systems thermal energy per unit temperature that is unavailable for doing useful work. Calculate the entropy change for.
Change in Entropy textDelta EntropytextEntropy in-textEntropy outtextEntropy gen. The inequality d q rev d q irrev together with the definition of entropy in terms of reversible heats gives. When we hold temperature constant an isothermal process and change one of the.
The change in entropy is inversely proportional to the temperature of the system.
Entropy Change For Melting Ice Heating Water Mixtures Carnot Cycle Of Heat Engines Physics Youtube
0 Comments